
Stainless Steel is a steel alloy containing chromium, well known for its corrosion resistance where durability and cleanliness are important. Here at Metals4U-Online, we only sell the highest quality stainless metals, and we ship direct to you! No order is cut 2 small! We’re HUGE!

Type 304 stainless steel (angle, etc) is a steel alloy containing chromium (18 to 20%)...

T-304 Stainless Steel Square Tube and Rectangular Tubing is produced by rolling the proper size...

Stainless Steel Flat bar is a steel alloy containing chromium, widely used in applications where...

Stainless Steel – a steel alloy containing chromium, well known for its corrosion resistance where...

A stainless-steel square bar is a steel bar with a square cross-section that’s made from...

Stainless steel T-304 is a chromium-nickel alloy sheet that is known for its strength, durability, and...

Type 304 Stainless Steel Sheet and Plate is a steel alloy containing chromium. T-304 sheet...

Stainless steel expanded metal is manufactured by slitting and stretching stainless steel sheets, producing diamond-shaped...
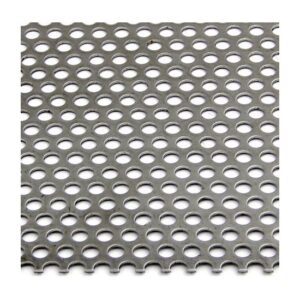
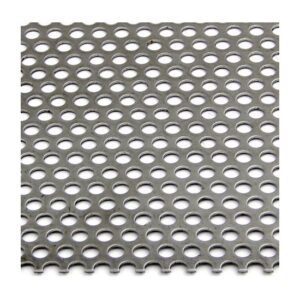
A stainless steel perforated sheet is a sheet of stainless steel that has been punched,...

Stainless Steel Pipe is a steel alloy containing chromium, widely used in applications where corrosion...

Stainless Steel Round Tube is the preferred choice where corrosion-resistance, durability, and sanitation are important...

Stainless steel all thread, also known as a stainless steel threaded rod, is a long,...

Metals4Uonline offers various options. Whether you need Dividers, J-Caps, Inside Corners, Outside Corners, or much more,...

Plank grating is a type of safety grating used in various settings to provide secure...

Our Stainless Steel Economy Value Packs are pre-packaged in 4ft lengths. Great for building up...

SSSQ414
SHOP THIS ITEM $8.85 – $16.61 Select options This product has multiple variants. The options may be chosen on the product page
SSSQ516
SHOP THIS ITEM $13.37 – $30.05 Select options This product has multiple variants. The options may be chosen on the product page
SSSQ438
SHOP THIS ITEM $10.83 – $28.34 Select options This product has multiple variants. The options may be chosen on the product page
SSSQ412
SHOP THIS ITEM $13.33 – $43.96 Select options This product has multiple variants. The options may be chosen on the product page
SSSQ458
SHOP THIS ITEM $13.55 – $52.22 Select options This product has multiple variants. The options may be chosen on the product page
SSSQ434
SHOP THIS ITEM $20.99 – $89.89 Select options This product has multiple variants. The options may be chosen on the product page
SSSQ478
SHOP THIS ITEM $42.55 – $196.30 Select options This product has multiple variants. The options may be chosen on the product page
SSSQ41
SHOP THIS ITEM $27.82 – $135.36 Select options This product has multiple variants. The options may be chosen on the product page
SSSQ4114
SHOP THIS ITEM $44.85 – $233.64 Select options This product has multiple variants. The options may be chosen on the product page
SSSQ4112
SHOP THIS ITEM $59.90 – $324.83 Select options This product has multiple variants. The options may be chosen on the product page
SSSQ4134
SHOP THIS ITEM $82.23 – $457.35 Select options This product has multiple variants. The options may be chosen on the product page
SSSQ42
SHOP THIS ITEM $108.10 – $611.68 Select options This product has multiple variants. The options may be chosen on the product page
SSSQ4212
SHOP THIS ITEM $260.00 – $1,385.00 Select options This product has multiple variants. The options may be chosen on the product page
SSFXM1216
SHOP THIS ITEM $40.00 – $111.00 Select options This product has multiple variants. The options may be chosen on the product page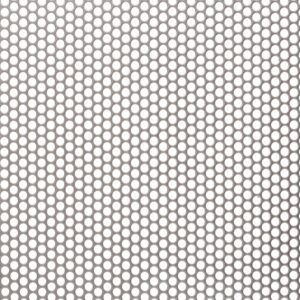
SSPE41618316F
SHOP THIS ITEM $96.24 – $268.72 Select options This product has multiple variants. The options may be chosen on the product page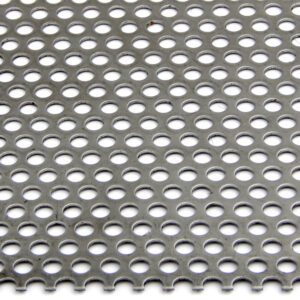
SSPE41631638
SHOP THIS ITEM $130.00 – $390.00 Select options This product has multiple variants. The options may be chosen on the product page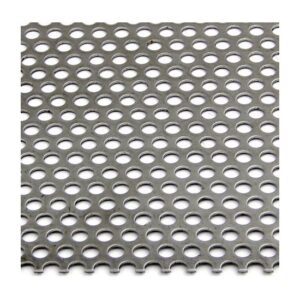
SSPE4161438
SHOP THIS ITEM $95.12 – $265.36 Select options This product has multiple variants. The options may be chosen on the product page
SSPI41440
SHOP THIS ITEM $13.00 – $25.00 Select options This product has multiple variants. The options may be chosen on the product page
(0.675 od x 0.091 wall x 0.493 id)*
SSPI43840
SHOP THIS ITEM $7.70 – $46.20 Select options This product has multiple variants. The options may be chosen on the product page
(0.840 od x 0.109 wall x 0.622 id)*
SSPI41240
SHOP THIS ITEM $6.05 – $36.30 Select options This product has multiple variants. The options may be chosen on the product page
(1.050 od x 0.113 wall x 0.824 id)*
SSPI43440
SHOP THIS ITEM $7.92 – $47.52 Select options This product has multiple variants. The options may be chosen on the product page
(1.315 od x 0.133 wall x 1.049 id)*
SSPI4140
SHOP THIS ITEM $11.44 – $68.64 Select options This product has multiple variants. The options may be chosen on the product page
(PVC Coated)
SSSH4244PVC
SHOP THIS ITEM $16.56 – $88.72 Select options This product has multiple variants. The options may be chosen on the product page
(PVC Coated)
SSSH4224PVC
SHOP THIS ITEM $14.03 – $58.38 Select options This product has multiple variants. The options may be chosen on the product page
(PVC Coated)
SSSH4204PVC
SHOP THIS ITEM $16.00 – $82.00 Select options This product has multiple variants. The options may be chosen on the product page
(PVC Coated)
SSSH4184PVC
SHOP THIS ITEM $18.40 – $110.80 Select options This product has multiple variants. The options may be chosen on the product page
SSAT41420
SHOP THIS ITEM $8.00 – $13.00 Select options This product has multiple variants. The options may be chosen on the product page
SSAT451618
SHOP THIS ITEM $8.80 – $14.60 Select options This product has multiple variants. The options may be chosen on the product page
SSAT43816
SHOP THIS ITEM $14.20 – $23.15 Select options This product has multiple variants. The options may be chosen on the product page
SSAT658
SHOP THIS ITEM $26.33 – $49.67 Select options This product has multiple variants. The options may be chosen on the product page
SSAT41213
SHOP THIS ITEM $18.00 – $33.00 Select options This product has multiple variants. The options may be chosen on the product page
SSAT45811
SHOP THIS ITEM $27.93 – $52.87 Select options This product has multiple variants. The options may be chosen on the product page
SSAT63410
SHOP THIS ITEM $73.53 – $144.07 Select options This product has multiple variants. The options may be chosen on the product page
SSAT4LH5/8-11
SHOP THIS ITEM $13.95 – $28.91 Select options This product has multiple variants. The options may be chosen on the product page
SSAT43410
SHOP THIS ITEM $37.53 – $72.07 Select options This product has multiple variants. The options may be chosen on the product page
SSAT41LH
SHOP THIS ITEM $65.00 – $96.00 Select options This product has multiple variants. The options may be chosen on the product page
SSAT41147
SHOP THIS ITEM $105.64 – $207.67 Select options This product has multiple variants. The options may be chosen on the product page
SSAT41126
SHOP THIS ITEM $129.00 – $255.00 Select options This product has multiple variants. The options may be chosen on the product page
SAN411211222I
SHOP THIS ITEM $24.24 Add to cart
SSAN411211222
SHOP THIS ITEM $24.24 Add to cart
SSFL4CAP
SHOP THIS ITEM $25.59 Add to cart
SSFL4FLDIVIVDER
SHOP THIS ITEM $24.10 Add to cart
SSPG4.75
SHOP THIS ITEM $199.41 – $388.81 Select options This product has multiple variants. The options may be chosen on the product page
SSPG167
SHOP THIS ITEM $190.18 – $370.36 Select options This product has multiple variants. The options may be chosen on the product page
SSPG11.75
SHOP THIS ITEM $271.44 – $445.73 Select options This product has multiple variants. The options may be chosen on the product page
(PVC Coated)
SSSH4164PVC
SHOP THIS ITEM $20.50 – $136.00 Select options This product has multiple variants. The options may be chosen on the product page
(PVC Coated)
SSSH4144PVC
SHOP THIS ITEM $22.50 – $160.00 Select options This product has multiple variants. The options may be chosen on the product page
PVC (Coated)
SSSH411
SHOP THIS ITEM $33.00 – $254.00 Select options This product has multiple variants. The options may be chosen on the product page
SSPE41638916
SHOP THIS ITEM $94.00 – $262.00 Select options This product has multiple variants. The options may be chosen on the product page
SSPE41431614
SHOP THIS ITEM $110.00 – $335.00 Select options This product has multiple variants. The options may be chosen on the product page
3/4" x 3/4" x 16ga.(.063") Stainless Steel Square Tube T-304 Mill Finish (...
SSST4343416MF
SHOP THIS ITEM $4.40 – $26.40 Select options This product has multiple variants. The options may be chosen on the product page
1" x 1" x 16ga.(.063") Stainless Steel Square Tube T-304 Mill Finish (...
SSST41116MF
SHOP THIS ITEM $4.60 – $27.59 Select options This product has multiple variants. The options may be chosen on the product page
3/4" x 3/4" x 16ga.(.063") Stainless Steel Square Tube T-304 180 Polished Finish
SSST4343416180P
SHOP THIS ITEM $7.81 – $46.86 Select options This product has multiple variants. The options may be chosen on the product page
1" x 1" x 11ga.(.120")Stainless Steel Square Tube T-304 Mill Finish (...
SSST41111MF
SHOP THIS ITEM $7.04 – $42.24 Select options This product has multiple variants. The options may be chosen on the product page
1" x 1" x 16ga.(.063") Stainless Steel Square Tube T-304 180 Polished Finish
SSST41116180P
SHOP THIS ITEM $4.95 – $29.70 Select options This product has multiple variants. The options may be chosen on the product page
180 Polished Stainless Steel Square Tube
SSST41111180P
SHOP THIS ITEM $7.59 – $45.54 Select options This product has multiple variants. The options may be chosen on the product page
1-1/4" x 1-1/4" x 16ga.(.063") Stainless Steel Square Tube T-304 ...
SSST411411416MF
SHOP THIS ITEM $9.48 – $56.89 Select options This product has multiple variants. The options may be chosen on the product page
1-1/4" x 1-1/4" x 16ga.(.063") Stainless Steel Square Tube T-304 180 ...
SSST411411416180P
SHOP THIS ITEM $12.22 – $73.32 Select options This product has multiple variants. The options may be chosen on the product page
1-1/4" x 1-1/4" x 11ga.(.120') Stainless Steel Square Tube ...
SSST411411411MF
SHOP THIS ITEM $16.94 – $101.64 Select options This product has multiple variants. The options may be chosen on the product page
1-1/4" x 1-1/4" x 11ga.(.120") Stainless Steel Square Tube T-304 180 ...
SSST411411411180P
SHOP THIS ITEM $12.54 – $75.24 Select options This product has multiple variants. The options may be chosen on the product page
1-1/2" x 1-1/2" x 16ga.(.063") Stainless Steel Square Tube T-304 180 ...
SSST411211216180P
SHOP THIS ITEM $14.30 – $85.80 Select options This product has multiple variants. The options may be chosen on the product page
1-1/2" x 1-1/2" x 16ga.(.063") Stainless Steel Square Tube T-304 ...
SSST411211216MF
SHOP THIS ITEM $15.40 – $92.40 Select options This product has multiple variants. The options may be chosen on the product page
1-1/2" x 1-1/2" x 11ga.(.120") Stainless Steel Square Tube T-304 180 ...
SSST411211211180P
SHOP THIS ITEM $17.34 – $104.02 Select options This product has multiple variants. The options may be chosen on the product page
1-1/2" x 1-1/2" x 11ga.(.120") Stainless Steel Square Tube T-304 ...
SSST411211211MF
SHOP THIS ITEM $13.20 – $79.20 Select options This product has multiple variants. The options may be chosen on the product page
1" x 2" x 16ga.(.063") Stainless Steel Rectangular Tube T-304 Mill Finish (...
SSST41216MF
SHOP THIS ITEM $7.70 – $46.20 Select options This product has multiple variants. The options may be chosen on the product page
1" x 2" x 16ga.(.063") Stainless Steel Rectangular Tube T-304 180 Polished Finish
SSST41216180P
SHOP THIS ITEM $12.68 – $76.08 Select options This product has multiple variants. The options may be chosen on the product page
1" x 2" x 11ga.(.120") Stainless Steel Rectangular Tube T-304 Mill Finish (...
SSST41211MF
SHOP THIS ITEM $13.75 – $82.50 Select options This product has multiple variants. The options may be chosen on the product page
2" x 2" x 16ga.(.063") Stainless Steel Square Tube T-304 Mill Finish (...
SSST42216MF
SHOP THIS ITEM $8.58 – $51.48 Select options This product has multiple variants. The options may be chosen on the product page
1" x 2" x 11ga.(.120") Stainless Steel Rectangular Tube T-304 180 Polished Finish
SSST41211180P
SHOP THIS ITEM $22.30 – $133.80 Select options This product has multiple variants. The options may be chosen on the product page
2" x 2" x 11ga.(.120") Stainless Steel Square Tube T-304 Mill Finish (...
SSST42211MF
SHOP THIS ITEM $15.09 – $90.55 Select options This product has multiple variants. The options may be chosen on the product page
2" x 2" x 16ga.(.063") Stainless Steel Square Tube T-304 180 Polished Finish
SSST42216180P
SHOP THIS ITEM $8.95 – $53.72 Select options This product has multiple variants. The options may be chosen on the product page
2" x 2" x 11ga.(.120") Stainless Steel Square Tube T-304 180 Polished Finish
SSST42211180P
SHOP THIS ITEM $17.05 – $102.30 Select options This product has multiple variants. The options may be chosen on the product page
SSRT6S375035
SHOP THIS ITEM $7.04 – $42.24 Select options This product has multiple variants. The options may be chosen on the product page
SSRT6S500065
SHOP THIS ITEM $13.75 – $82.50 Select options This product has multiple variants. The options may be chosen on the product page
SSRT6S750065
SHOP THIS ITEM $17.60 – $105.60 Select options This product has multiple variants. The options may be chosen on the product page
SSRT6S1000065
SHOP THIS ITEM $21.23 – $127.38 Select options This product has multiple variants. The options may be chosen on the product page
SSRT6S1250065
SHOP THIS ITEM $33.77 – $202.62 Select options This product has multiple variants. The options may be chosen on the product page
SSRT4W250035
SHOP THIS ITEM $4.40 – $26.40 Select options This product has multiple variants. The options may be chosen on the product page
SSRT4w375035
SHOP THIS ITEM $4.18 – $25.08 Select options This product has multiple variants. The options may be chosen on the product page
SSRT4W500035
SHOP THIS ITEM $5.50 – $33.00 Select options This product has multiple variants. The options may be chosen on the product page
SSRT4W625035
SHOP THIS ITEM $7.70 – $46.20 Select options This product has multiple variants. The options may be chosen on the product page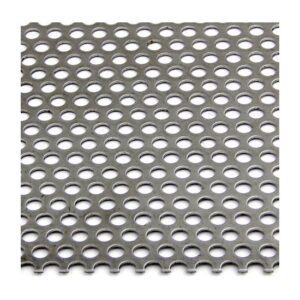
SSPE41438916
SHOP THIS ITEM $110.16 – $310.48 Select options This product has multiple variants. The options may be chosen on the product page
SSPE4111438
SHOP THIS ITEM $180.48 – $521.44 Select options This product has multiple variants. The options may be chosen on the product page
SSPE411121116
SHOP THIS ITEM $135.00 – $415.00 Select options This product has multiple variants. The options may be chosen on the product page
(PVC Coated)
SSSH42B16
SHOP THIS ITEM $24.00 – $135.00 Select options This product has multiple variants. The options may be chosen on the product page
(PVC Coated)
SSSH42B14
SHOP THIS ITEM $26.00 – $159.00 Select options This product has multiple variants. The options may be chosen on the product page
(PVC Coated)
SSSH42B11
SHOP THIS ITEM $33.00 – $244.00 Select options This product has multiple variants. The options may be chosen on the product page
3/16" Stainless Steel Round T-316
SSRO6316
SHOP THIS ITEM $5.00 – $8.50 Select options This product has multiple variants. The options may be chosen on the product page
SSRO418
SHOP THIS ITEM $5.93 – $18.00 Select options This product has multiple variants. The options may be chosen on the product page
SSRO3316
SHOP THIS ITEM $5.00 – $19.60 Select options This product has multiple variants. The options may be chosen on the product page
SSRO4316
SHOP THIS ITEM $11.89 – $67.28 Select options This product has multiple variants. The options may be chosen on the product page
SSRO314
SHOP THIS ITEM $5.00 – $40.00 Select options This product has multiple variants. The options may be chosen on the product page
SSRO414
SHOP THIS ITEM $2.12 – $22.84 Select options This product has multiple variants. The options may be chosen on the product page
SSRO614
SHOP THIS ITEM $5.00 – $21.00 Select options This product has multiple variants. The options may be chosen on the product page
SSRO3516
SHOP THIS ITEM $2.61 – $18.29 Select options This product has multiple variants. The options may be chosen on the product pageStainless Steel, is also described as inox steel (derived from French: “inoxydable” meaning stainless), is a steel alloy. The major difference between stainless steel and ordinary (carbon) steel is that the former does not undergo corrosion when exposed to water or air while the latter does. Therefore, stainless steel finds use in applications that not only need the properties of steel but also require high resistance to corrosion.
During the steel making process, various elements are added to ordinary steel when it is still in its molten state. The elements added, as well as the quantities of those elements, will change the properties of steel. If, during the process of steel production, the amount of chromium added exceeds 10.5% of the total mass of the alloy, the resultant steel alloy is stainless steel.
Stainless Steel is highly resistant to corrosion because of the large amount of chromium that it contains. This chromium reacts with the oxygen present in the atmosphere to form a passive layer of chromium oxide. As a result of the formation of this layer, oxygen in the atmosphere is unable to reach the actual steel surface; this protects the metal alloy from rusting or corroding. However, for this to happen, not only should sufficient amount of chromium be present, so should sufficient amount of oxygen. Therefore, in environments having low oxygen levels, stainless steel might still undergo corrosion.
The property of non-corrosion of an alloy of iron and chromium was first discovered by Pierre Berthier, who was a French metallurgist, in the year 1821. However, in the 19th century, metallurgists were not able to actually produce a steel alloy that had high levels of chromium and low levels of carbon. The alloys that they produced had high levels of carbon and were too brittle. It wasn’t until the latter part of the 19th century and earlier part of the 20th century, however, that a number of researchers, like the Frenchman Leon Guillet and others in Europe and the United States of America, managed to create alloys which, today, we would consider as stainless steel. And all of this started when a German chemist called Hans Goldschmidt developed a thermite process to produce chromium that was carbon-free. By the third decade of the twentieth century, mass production of stainless steel had set in.
* Prices subject to change without notice due to the Metals Market fluctuations.
* All ‘value length’ and ‘value pack’ item measurements are subject to a “mill tolerance”. A product may be produced several thousandths of an inch, either over or under the stated thickness, and still be within “mill tolerance”. This does NOT apply to Cut To Size items. Those will be +.125″, -0.00″ tolerances.

CALIFORNIA PROPOSITION 65
This product can expose you to chemicals which are known to the State of California to cause cancer, birth defects or other reproductive harm. For more information, go to www.P65Warnings.ca.gov
Prices subject to change without notice due to the Metals Market fluctuations. | All ‘value length’ and ‘value pack’ item measurements are subject to a “mill tolerance”. | A product may be produced several thousandths of an inch, either over or under the stated thickness, and still be within “mill tolerance”. This does NOT apply to Cut To Size items. Those will be +.125”, -0.00” tolerances.

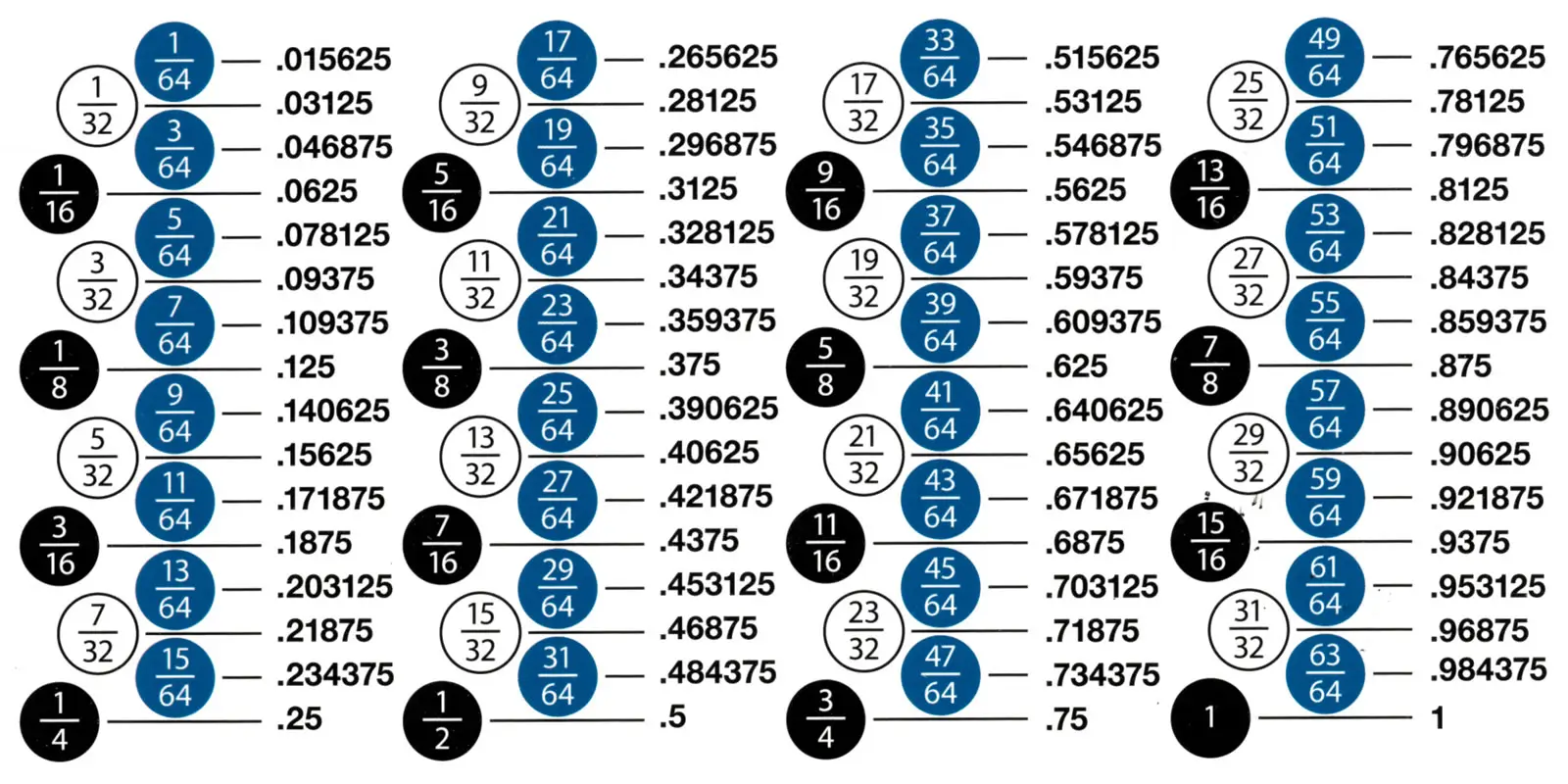

|
Pipe Size* |
O.D.
(in.) |
Schedule (10, 40, 80)
Wall Thickness (in.)** |
|||||
|---|---|---|---|---|---|---|---|
| Sch.10 | Sch.40 | Sch.80 | |||||
| Wall (in) | I.D. (in) | Wall (in) | I.D. (in) | Wall (in) | I.D. (in) | ||
| 1/8" | 0.41 od | 0.049 in | 0.312 id | 0.07 in | 0.269 id | ||
|
Weight (lbs/ft.) |
Steel | 0.183 lbs/ft | 0.247 lbs/ft | ||||
| Stainless | |||||||
| Aluminum | |||||||
| 1/4" | 0.54 od | 0.065 in | 0.410 id | 0.090 in | 0.364 id | 0.119 in | 0.302 id |
|
Weight (lbs/ft.) |
Steel | 0.333 lbs/ft | 0.429 lbs/ft | 0.535 lbs/ft | |||
| Stainless | |||||||
| Aluminum | 0.147 lbs/ft | ||||||
| 3/8" | 0.675 od | 0.065 in | 0.545 id | 0.091 in | 0.493 id | 0.126 in | 0.423 id |
|
Weight (lbs/ft.) |
Steel | 0.420 lbs/ft | 0.570 lbs/ft | 0.740 lbs/ft | |||
| Stainless | |||||||
| Aluminum | 0.196 lbs/ft | ||||||
| 1/2" | 0.840 od | 0.083 in | 0.674 id | 0.109 in | 0.622 id | 0.147 in | 0.546 id |
|
Weight (lbs/ft.) |
Steel | 0.670 lbs/ft | 0.850 lbs/ft | 1.090 lbs/ft | |||
| Stainless | |||||||
| Aluminum | 0.294 lbs/ft | 0.384 lbs/ft | |||||
| 3/4" | 1.050 od | 0.083 in | 0.884 id | 0.113 in | 0.824 id | 0.154 in | 0.742 id |
|
Weight (lbs/ft.) |
Steel | 0.86 lbs/ft | 1.13 lbs/ft | 1.48 lbs/ft | |||
| Stainless | |||||||
| Aluminum | 0.391 | 0.520 | |||||
| 1" | 1.315 od | 0.109 in | 1.097 id | 0.133 in | 1.049 id | 0.179 in | 0.957 id |
|
Weight (lbs/ft.) |
Steel | 1.41 lbs/ft | 1.68 lbs/ft | 2.17 lbs/ft | |||
| Stainless | |||||||
| Aluminum | 0.481 lbs/ft | 0.581 lbs/ft | 0.781 lbs/ft | ||||
| 1-1/4" | 1.66 od | 0.109 in | 1.442 id | 0.140 in | 1.380 id | 0.191 in | 1.278 id |
|
Weight (lbs/ft.) |
Steel | 1.81 lbs/ft | 2.27 lbs/ft | 3.00 lbs/ft | |||
| Stainless | |||||||
| Aluminum | 0.618 lbs/ft | 0.785 lbs/ft | 1.040 lbs/ft | ||||
| 1-1/2" | 1.90 od | 0.109 in | 1.682 id | 0.145 in | 1.610 id | 0.200 in | 1.500 id |
|
Weight (lbs/ft.) |
Steel | 2.09 lbs/ft | 2.72 lbs/ft | 3.63 lbs/ft | |||
| Stainless | |||||||
| Aluminum | 0.714 lbs/ft | 0.939 lbs/ft | 1.260 lbs/ft | ||||
| 2" | 2.375 od | 0.109 in | 2.157 id | 0.154 in | 2.067 id | 0.218 in | 1.939 id |
|
Weight (lbs/ft.) |
Steel | 2.64 lbs/ft | 3.66 lbs/ft | 5.03 lbs/ft | |||
| Stainless | |||||||
| Aluminum | 0.903 lbs/ft | 1.260 lbs/ft | 1.740 lbs/ft | ||||
| 2-1/2" | 2.875 od | 0.120 in | 2.635 id | 0.203 in | 2.469 id | 0.276 in | 2.323 id |
|
Weight (lbs/ft.) |
Steel | 3.53 lbs/ft | 5.80 lbs/ft | 7.67 lbs/ft | |||
| Stainless | |||||||
| Aluminum | 1.209 lbs/ft | 2.000 lbs/ft | |||||
| 3" | 3.50 od | 0.120 in | 3.26 id | 0.216 in | 3.068 id | 0.30 in | 2.90 id |
|
Weight (lbs/ft.) |
Steel | 4.34 lbs/ft | 7.58 lbs/ft | 10.26 lbs/ft | |||
| Stainless | |||||||
| Aluminum | 1.483 lbs/ft | 2.620 lbs/ft | 3.55 lbs/ft | ||||
| 3-1/2" | 4.00 od | 0.120 in | 3.76 id | 0.226 in | 3.550 id | 0.318 in | 3.360 id |
|
Weight (lbs/ft.) |
Steel | 4.98 lbs/ft | 9.12 lbs/ft | 12.52 lbs/ft | |||
| Stainless | |||||||
| Aluminum | 3.150 lbs/ft | 4.33 lbs/ft | |||||
| 4" | 4.50 od | 0.120 in | 4.26 id | 0.237 in | 4.026 id | 0.337 in | 3.826 id |
|
Weight (lbs/ft.) |
Steel | 5.62 lbs/ft | 10.80 lbs/ft | 15.00 lbs/ft | |||
| Stainless | |||||||
| Aluminum | 1.922 lbs/ft | 3.730 lbs/ft | 5.180 lbs/ft | ||||
| 5" | 5.563 od | 0.134 in | 5.295 id | 0.258 in | 5.047 id | 0.375 in | 4.813 id |
|
Weight (lbs/ft.) |
Steel | 7.78 lbs/ft | 14.63 lbs/ft | 20.80 lbs/ft | |||
| Stainless | |||||||
| Aluminum | 2.660 lbs/ft | 5.050 lbs/ft | 7.190 lbs/ft | ||||
| 6" | 6.625 od | 0.134 in | 6.357 id | 0.280 in | 6.065 id | 0.432 in | 5.761 id |
|
Weight (lbs/ft.) |
Steel | 9.30 lbs/ft | 18.99 lbs/ft | 28.60 lbs/ft | |||
| Stainless | |||||||
| Aluminum | 3.181 lbs/ft | 6.560 lbs/ft | 9.880 lbs/ft | ||||
| 8" | 8.625 od | 0.148 in | 8.329 id | 0.322 in | 7.981 id | 0.500 in | 7.625 id |
|
Weight (lbs/ft.) |
Steel | 13.41 lbs/ft | 28.58 lbs/ft | 43.43 lbs/ft | |||
| Stainless | |||||||
| Aluminum | 9.88 lbs/ft | 15.010 lbs/ft | |||||
|
*Nominal sizes apply - Pipe Size is the generic Industry Size Standard for reference only **Tolerances may vary slightly from each manufacturer |
|||||||

| Ga# | Sheet Steel | Galvanized Steel | Stainless Steel | Aluminum | Strip & Tubing | ||||
|---|---|---|---|---|---|---|---|---|---|
|
Ga (in.) |
Wgt (lb/ft2) |
Ga (in.) |
Wgt (lb/ft2) |
Ga (in.) |
Wgt (lb/ft2) |
Ga (in.) |
Wgt (lb/ft2) |
Ga (in.) |
|
| 38 | 0.0060 | 0.0062 | 0.0040 | ||||||
| 37 | 0.0064 | 0.0066 | 0.0045 | ||||||
| 36 | 0.0067 | 0.0070 | 0.0050 | 0.004 | |||||
| 35 | 0.0075 | 0.0078 | 0.0056 | 0.005 | |||||
| 34 | 0.0082 | 0.0086 | 0.0063 | 0.007 | |||||
| 33 | 0.0090 | 0.0094 | 0.0071 | 0.008 | |||||
| 32 | 0.0097 | 0.0102 | 0.0080 | 0.009 | |||||
| 31 | 0.0105 | 0.0109 | 0.0089 | 0.010 | |||||
| 30 | 0.0120 | 0.500 | 0.016 | 0.656 | 0.0125 | 0.0100 | 0.141 | 0.012 | |
| 29 | 0.0135 | 0.563 | 0.017 | 0.719 | 0.0141 | 0.0113 | 0.160 | 0.013 | |
| 28 | 0.0149 | 0.625 | 0.019 | 0.781 | 0.0156 | 0.0126 | 0.178 | 0.014 | |
| 27 | 0.0164 | 0.688 | 0.020 | 0.844 | 0.0172 | 0.0142 | 0.200 | 0.016 | |
| 26 | 0.0179 | 0.750 | 0.022 | 0.906 | 0.0187 | 0.756 | 0.0159 | 0.224 | 0.018 |
| 25 | 0.0209 | 0.875 | 0.025 | 1.031 | 0.0219 | 0.0179 | 0.253 | 0.020 | |
| 24 | 0.0239 | 1.000 | 0.028 | 1.156 | 0.0250 | 1.008 | 0.0201 | 0.284 | 0.022 |
| 23 | 0.0269 | 1.125 | 0.031 | 1.281 | 0.0281 | 0.0226 | 0.319 | 0.025 | |
| 22 | 0.0299 | 1.250 | 0.034 | 1.406 | 0.0312 | 1.26 | 0.0253 | 0.357 | 0.028 |
| 21 | 0.0329 | 1.375 | 0.037 | 1.531 | 0.0344 | 0.0285 | 0.402 | 0.032 | |
| 20 | 0.0359 | 1.500 | 0.040 | 1.656 | 0.0375 | 1.512 | 0.0320 | 0.452 | 0.035 |
| 19 | 0.0418 | 1.750 | 0.046 | 1.906 | 0.0437 | 0.0359 | 0.507 | 0.042 | |
| 18 | 0.0478 | 2.000 | 0.052 | 2.156 | 0.0500 | 2.016 | 0.0403 | 0.569 | 0.049 |
| 17 | 0.0538 | 2.250 | 0.058 | 2.406 | 0.0562 | 0.0453 | 0.639 | 0.058 | |
| 16 | 0.0598 | 2.500 | 0.064 | 2.656 | 0.0625 | 2.52 | 0.0508 | 0.717 | 0.065 |
| 15 | 0.0673 | 2.813 | 0.071 | 2.969 | 0.0703 | 0.0571 | 0.806 | 0.072 | |
| 14 | 0.0747 | 3.125 | 0.079 | 3.281 | 0.0781 | 3.15 | 0.0641 | 0.905 | 0.083 |
| 13 | 0.0897 | 3.750 | 0.093 | 3.906 | 0.0937 | 0.0720 | 1.016 | 0.095 | |
| 12 | 0.1046 | 4.375 | 0.108 | 4.531 | 0.1094 | 4.41 | 0.0808 | 1.140 | 0.109 |
| 11 | 0.1196 | 5.000 | 0.123 | 5.156 | 0.1250 | 5.04 | 0.0907 | 1.280 | 0.120 |
| 10 | 0.1345 | 5.625 | 0.138 | 5.781 | 0.1406 | 5.67 | 0.1019 | 1.438 | 0.134 |
| 9 | 0.1495 | 6.250 | 0.153 | 6.406 | 0.1562 | 0.1144 | 1.614 | 0.148 | |
| 8 | 0.1644 | 6.875 | 0.168 | 7.031 | 0.1719 | 6.93 | 0.1285 | 1.813 | 0.165 |
| 7 | 0.1793 | 7.500 | 0.1875 | 7.871 | 0.1443 | 2.036 | 0.180 | ||
| 6 | 0.1943 | 8.125 | 0.2031 | 0.1620 | 2.286 | 0.203 | |||
| 5 | 0.2092 | 8.750 | 0.2187 | 0.1819 | 0.220 | ||||
| 4 | 0.2242 | 9.375 | 0.2344 | 0.2043 | 0.238 | ||||
| 3 | 0.2391 | 10.00 | 0.2500 | 0.2294 | 0.259 | ||||
| 2 | 0.2656 | 0.2576 | 0.284 | ||||||
| 1 | 0.2812 | 0.2893 | 0.300 | ||||||